|
快速叶绿素荧光(OJIP)可作为监测植物在非生物胁迫下光合生理状态的有效工具
欢迎点击「汉莎科学仪器」↑关注我们! 
摘要在自然条件下生活的植物会受到许多干扰光合作用过程的不利因素的影响,导致生长、发育和产量的下降。叶绿素a荧光光谱(ChlF)的研究为叶片光化学效率研究提供了一条新的途径。具体地说,对荧光信号的分析可获取PSII反应中心、捕光天线复合体以及PSII供体侧/受体侧的状态和功能的详细信息。在这里,我们回顾了快速ChlF技术(OJIP & JIP-test)分析光合反应对环境胁迫的相关成果,并讨论了这一创新方法的潜在科学和实际应用。最近便携式设备(Handy PEA & M-PEA, Hansatech Instruments)的出现,特别是在作物表型分型和监测方面,大大扩展了ChlF技术的潜在应用。 关键词 Chlorophyll fluorescence、JIP-test、Photosynthesis、Photosystem II、Quantum efficiency、Stress detection

缩写
| Absorption flux | 吸收通量 | | Chlorophyll | 叶绿素 | | Chlorophyll fluorescence | 叶绿素荧光 | | Cross section of the sample | 样品横截面 | | Cytochrome b6f | 细胞色素b6f | | Delayed (chlorophyll) fluorescence | 延迟(叶绿素)荧光 | | Drought factor index | 干旱因子指数 | | Light-harvesting complex (of PSII) | PSII捕光色素复合体 | | Oxygen-evolving complex | 放氧复合体 | | Excited PSII reaction center | 激发的PSII反应中心 | | PSI reaction center | PSI反应中心 | | Photosynthetically active radiation | 光合有效辐射 | | Plastocyanin | 质体蓝素 | | Principal component analysis | 主成分分析 | | Prompt (chlorophyll) fluorescence | 瞬时(叶绿素)荧光 | | Pheophytin | 去镁叶绿素 | | Plastoquinone | 质体醌 | | Photosystem I, II | 光系统I, II | | Primary plastoquinone electron acceptor of PSII | PSII初级质体醌电子受体 | | Secondary plastoquinone electron acceptor | 次级质体醌电子受体 | | Reaction center | 反应中心 | | Reactive oxygen species | 活性氧 |
在21世纪,全球农业必须生产更多的粮食来维持不断增长的人口(Beddington et al. 2012)。然而,这一目标受到人为气候变化的威胁,这种变化有可能显著降低受影响地区的粮食产量(Lobell et al.2008)。最近的研究表明,叶绿素荧光(ChlF)测量可以为改进全球农业生产力模型提供独特的基准,提高气候变化情景下作物产量预测的可靠性(Guanter et al. 2014; Malaspina et al. 2014)。更广泛地说,ChlF技术正在成为农业、环境和生态研究中的一个非常强大的工具(Gottardiniet al. 2014)。它的一个主要优点是ChlF技术是一种非侵入性的工具,允许科学家在不破坏被测样品的情况下获得光合过程的丰富信息。在自然条件下,植物受到许多不利的环境胁迫因子的影响。这些会破坏光合器官,导致植物生产力和总产量下降。光合作用对环境胁迫特别敏感(Kalaji et al. 2012),使光合测量成为植物胁迫研究的重要组成部分。然而,传统的方法,甚至是技术上先进的方法,如通过气体交换(CO2、H2O和O2)测量光合速率,需要耗费大量时间和人力,且提供的有关整体光合功能的信息并不完整。相比之下,ChlF测量是一种简单、无损、廉价和快速的工具,可用于分析光依赖性光合反应和间接评估同一样本组织中的叶绿素含量(Govindjee 1995; Papageorgiou & Govindjee 2011; Stirbet & Govindjee 2011, 2012)。ChlF方法的这些技术优势使其成为植物育种家(例如作物表型和监测)、生物技术学家、植物生理学家、林业工作者、生态学家和环境学家的流行技术。

关键的是,从植物胁迫研究的角度来看,ChlF测量还提供了有关植物生理状况的间接信息。通过分析叶绿素荧光(ChlF)诱导曲线,可以评估光系统II(PSII)和光合电子传递链的生理状况。它还提供了光依赖的光化学反应和光无关的生化反应的相关信息。总的来说,ChlF测量直接或间接地与依赖光的光合反应的所有阶段有关,包括水的光解、电子传递、类囊体膜上pH梯度的形成、ATP合成以及光合机构的一般生物能条件等(Bernát et al. 2012)。 许多ChlF技术和应用现在已经开发出来,为植物光合作用研究提供了丰富的技术手段。本文综述了连续激发式荧光仪测量的快速叶绿素荧光动力学曲线(OJIP)的相关学术成果。这些研究是通过开发一个可靠的数学模型JIP-test(Strasser et al. 2004),允许分析在不到1s内发生的荧光变化。此类分析提供了关于PSII反应中心、天线以及PSII的供体和受体侧的状态和功能的详细信息。综述了胁迫因子对光化学过程的影响,对快速ChlF动力学和相关生物物理参数的变化规律。暗适应叶片照光后可获得多相叶绿素荧光诱导曲线(O–J–I–P-瞬变)(图1)。曲线的轨迹提供了有关光合机构结构和功能的大量信息(Kautsky & Hirsch 1931; Schreiber et al. 1994)。
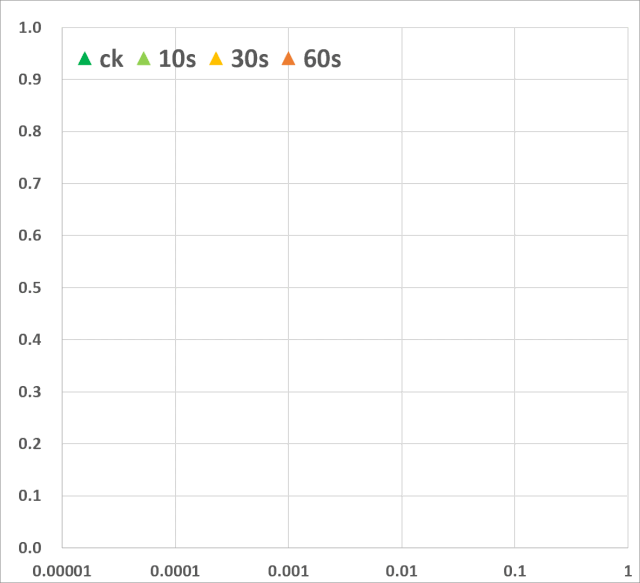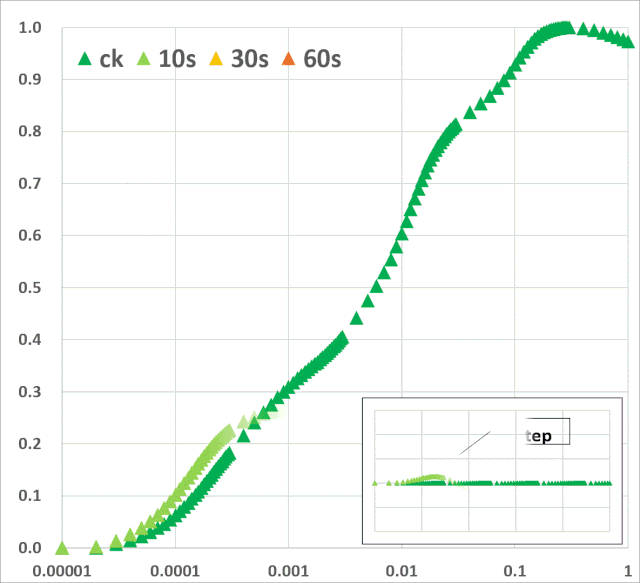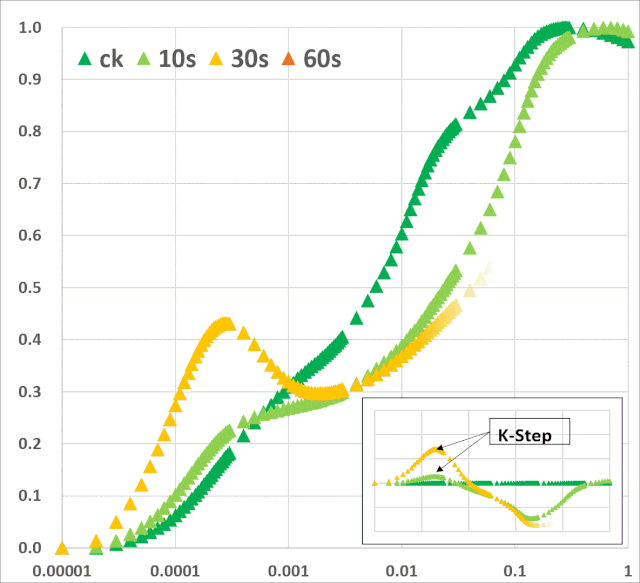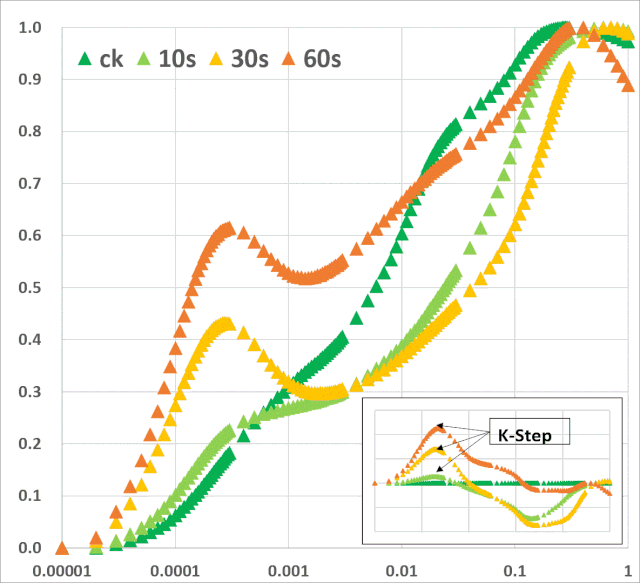
JIP-test是基于多相快速叶绿素荧光的上升阶段,用于研究光依赖性反应与ChlF的相关性。它基于类囊体膜的“能量流”理论(Strasser et al. 2000)。这个理论可以用简单的代数方程来计算,代表每一个被检测的捕光复合体的总能量流入和流出之间的平衡,并提供关于吸收能量的可能分配的信息。利用这些方程,可以描述PSII复合体之间的能量通信(也称为“聚集grouping”或“连通性connectivity”和“总体分组概率overall grouping probability”)(Stirbet 2013)。 JIP-test(OJIP)的名称来源于ChlF信号形成的感应曲线上的特定位点(图1):这些位点对应于PSII原初电子受体(Pheo)和QA的逐渐还原。诱导曲线的形状取决于PSII各组分间的聚集性(L-band)(Tsimilli-Michaeland Strasser 2013)和电子供体OEC→P680+以及QA-电子的接收之间的平衡(K-band)(Strasser et al. 2005)。 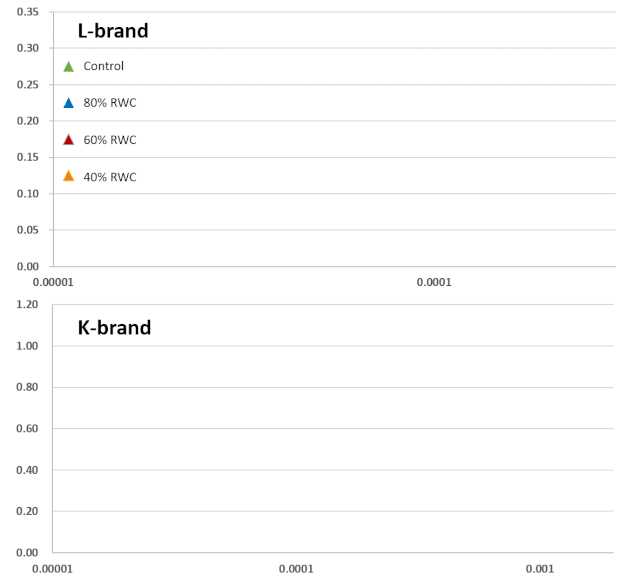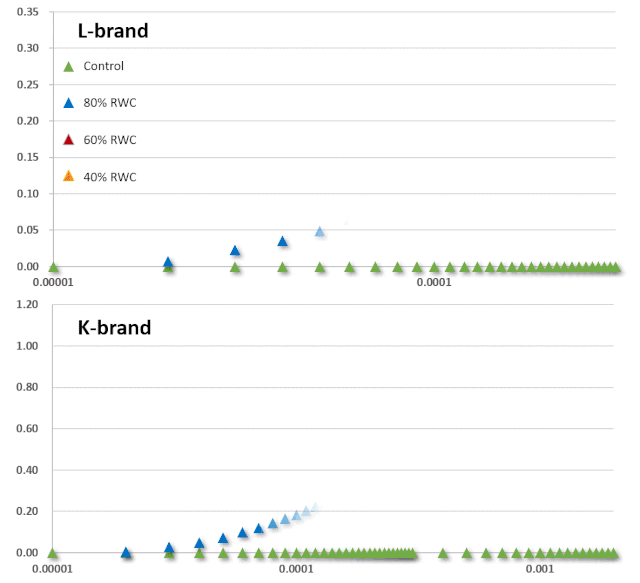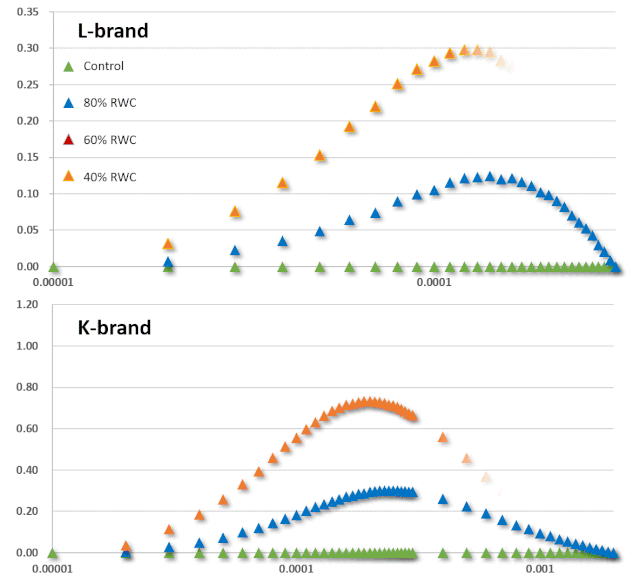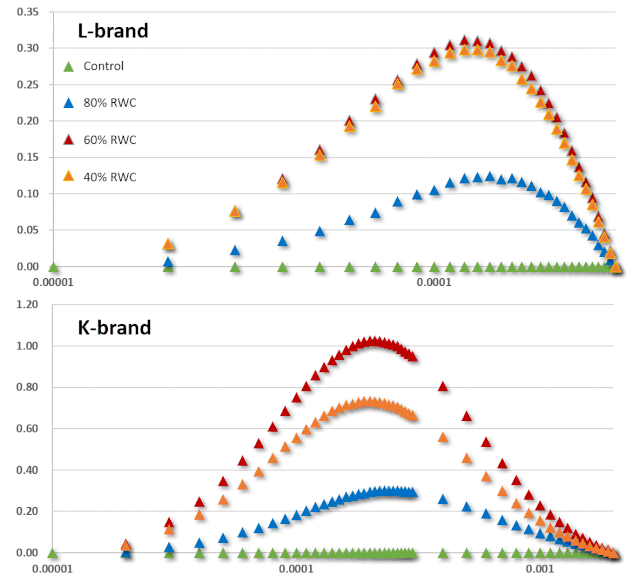
O~J相的荧光上升阶段与部分PSII反应中心的闭合相关,反应了QA的还原水平,其还原程度取决于捕获速率以及QA被QB和其余电子传递链成员氧化的速率。 诱导曲线的J~I相与次级电子受体QB、PQ、Cyt b6f和PC的还原程度相关。诱导曲线的I~P相的上升通常归因于PSI受体侧电子受体(铁氧还原蛋白、中间受体和NADP)的还原。 高温、强光、缺氮或干旱胁迫会抑制放氧复合体OEC并阻碍OEC与酪氨酸之间的电子传递(Guha et al. 2013)。胁迫条件下,在ChlF诱导曲线200~300μs范围内会出现一个波峰——K-band,表明OEC已受到破坏。
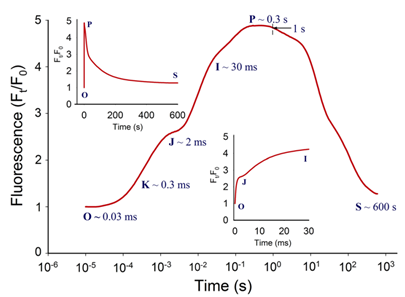
图1:典型的植物叶绿素荧光多相动力学曲线(主图),曲线以对数时间刻度(10μs~600s)绘制。左上部插图显示了按常规时间标度绘制的相同曲线。右下方插图按常规时间标度绘制的OJIP瞬态(0-30ms)的初始部分。时间标记是指JIP-test用于计算结构和功能参数的选定时间点。 表征PAR能量吸收和电子传递的JIP-test参数可主要分为以下四组:(1)基本测量值和计算值[荧光(Ft)、可变荧光(Vt)值和初始斜率等];(2)量子产率和概率;(3)能量通量;和(4)性能指数。表征能量通量的生物物理参数分为specific和phenomenological两大类。specific参数按反应中心(RC)计算,phenomenological参数按样品截面(CS)计算。性能指数(PI)由几个独立参数的乘积计算得出,分别包括反应中心的密度、初级光化学反应的量子效率和电子传递中激发能的转换(Strasser et al. 2000, 2004, 2010; Zushi et al. 2012)。性能指数被创建为非特定参数,主要用于实际应用,如筛选在田间条件下增强的应力耐受性(Srivastava et al. 1999; Strasser et al. 2004; Brestic & Zivcak 2013)。叶绿素荧光动力学也可以用来揭示光合机构的PSII异质性。PSII在天线色素和还原侧方面具有天然异质性。天线异质性包括天线尺寸和天线色素分子组分间的连通性(或聚集性)差异。PSII反应中心基于天线尺寸可分为三类:alpha (α), beta (β)和gamma (γ) (Melis & Homann 1976),其主要区别在于天线寿命和伴生叶绿素的数量。还原侧的异质性主要与从QA开始电子传递的能力有关。可将电子由QA传递给QB的反应中心命名为可还原QB的反应中心(QB reducing centers),而不具备此能力的反应中心称之为不可还原QB的反应中心(QB non-reducing centers)。Jajoo(2013)回顾了PSII异质性的具体特征。近期研究表明,高温(Mathur et al. 2011b),高盐(Mehta et al. 2010a)以及如多环芳烃(PAH)等污染物(Tomarand Jajoo 2013, 2014)胁迫下均会引起PSII异质性的变化。PSII异质性的变化可能与活跃/不活跃反应中心的数量有关,在各种胁迫条件下活跃的α反应中心转换为非活跃的β和γ反应中心,同时不可还原QB的反应中心数量也随之增多。在下面的章节中,我们回顾了ChlF动力学可以作为气候变化和人类活动(如高温和低温、干旱、盐分、营养缺乏和重金属)负面影响的有效指标的证据。 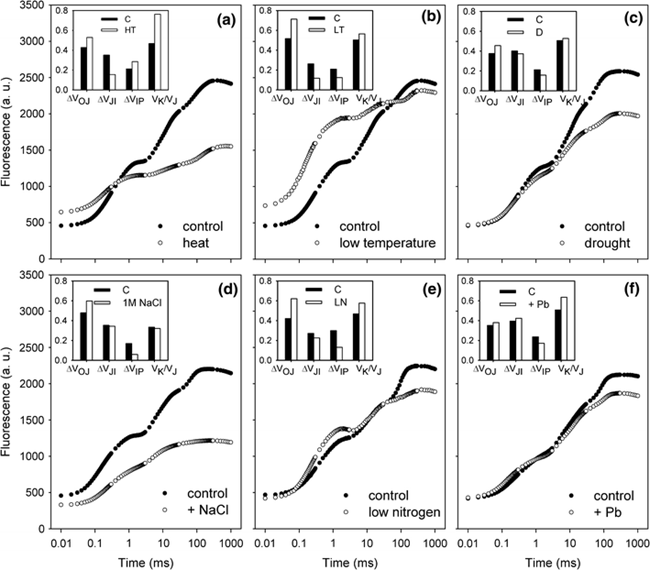
图2:不同胁迫条件下小麦(Triticum sp.L.)叶绿素荧光的O(K)JIP瞬态与非胁迫下的比较。插入显示了O-J相(∆VOJ)、J-I相(∆VJI)、I-P相(∆VIP)的相对可变荧光振幅的变化,以及0.3 ms可变荧光(VK/VJ)与2ms可变荧光比值(VJ)的变化,作为PSII供体侧限制(K-band)的指标。各图显示了相对于非胁迫状态下植物(control,C)的瞬时荧光曲线:a热胁迫(高温胁迫8h,中度光化光照射,叶片温度约40℃);b低温胁迫10d(10/6℃:日间/夜间);c重度干旱胁迫(停止灌溉后12d,叶片含水量约60%);d盐胁迫(NaCl);e氮缺乏胁迫(低氮,LN);f铅胁迫。 气候变化可能会增加植物的热胁迫,限制生产力和生物量的积累。光合作用是植物细胞过程中对高温最敏感的过程(Sharkey & Schrader 2006),高温会导致PSII电子受体还原-氧化特性发生变化,并降低两个光系统中光合电子传输的效率(Mathur et al. 2014)。热胁迫会影响ChlF参数的值(图2a)。例如苹果Malus x domestica Borkh在高温胁迫下,其QA-/RC和QB-/QA-的比率均产生下降,同时PSII最大量子产率(Fv/Fm)降低而最小荧光Fo升高(Chen et al. 2009; Brestic et al. 2013)。高温胁迫同样会对O-J-I-P曲线的形状产生影响,会导致Fm的降低和Fo升高。Fo的升高可能是由于捕光色素复合体LHC II从PSII复合体上解离、PSII光化学反应的失活或还原的电子受体QA至QB电子流传递受到抑制而导致的(Mathur et al. 2011a)。例如,由菠菜和水稻中观察到的Fo升高归因于LHC II与PSII复合体的不可逆解离和PSII的部分可逆性失活(Yamane et al. 1997)。Fm的降低可能与叶绿素蛋白的变性有关(Yamane et al. 1997)。K峰(300μs)是热应激一个极佳的指示指标,可用于指示放氧复合体OEC的解离和去镁叶绿素Pheo与初级电子受体QA间的电子传递情况(Strasser et al. 2000; Lazár 2006)。在小麦中,35℃处理时对净光合速率未产生影响,而当45℃处理时则对OEC产生了不可逆的损伤(Schreiber et al. 2012)。K峰出现的直接原因是电子由P680向PSII电子受体的流出量远超于电子由PSII供体侧向P680的流入量。同时K峰也会受到光系统II之间能量关系变化的影响。FK/FJ比率的增大表明热胁迫抑制了OEC的电子供应(Srivastava & Strasser 1995)。快速ChlF技术也是监测PSII热稳定性有效方法。最有效的方法是评估临界温度,即在临界温度以上观测相应参数的快速增加或减少(Brestic & Zivcak 2013)。在作物育种中,一些基因型可以作为增强耐热性的供体。以热处理菜豆(Phaseolus vulgaris L.)品系为例,通过ChlF诱导的变化来监测其胁迫反应和恢复状况,并应用JIP-test进行分析(Stefanov et al. 2011)。Brestic et al. (2012)利用快速ChlF动力学方法对30个不同地理来源冬小麦基因型的PSII热稳定性进行了研究。Gautum et al. (2014)证明ChlF法在硬粒小麦基因型筛选中比常规方法(如收获指数、籽粒灌浆等)更有效。在某些纬度地区,低温是限制作物产量的主要因素(Yang et al. 2009)。在北半球冬季和早春的低温通常伴随着强光照现象,在这种环境条件下,会导致植物类囊体结构退化和光依赖性的光合反应的扭曲(Suzuki et al. 2011)。冷胁迫同样会影响ChlF参数(图2b)。例如,低温胁迫下苦瓜(Momordica charantia L.)植株的叶绿素含量、PSII供体侧OEC效率、光化学猝灭和开放的PSII反应中心效率均降低(Yang et al. 2009)。某些具有极佳低温耐受性的物种表现出较少的PSII光抑制。例如,低温胁迫下豌豆植株的ChlF参数只有极小的变化(Strauss et al. 2006; Strebet al. 2008)。干旱胁迫对光合器官的影响是众所周知的。在中等干旱强度下它们通常开始主要是气孔效应,严重或长期干旱胁迫最终会导致代谢和结构性变化(Jedmowski et al. 2013)。这种最终的变化也与光保护和抗氧化功能和途径的增强有关(Chaves et al. 2009)。与PSI相比,PSII具有较高的抗缺水能力,因此只有在极端干旱的情况下才会产生负面影响(Lauriano et al. 2006)。ChlF测量表明,在干旱条件下,通过调节光系统之间的能量分布和激活交替电子流,增强了PSII和PSI光化学的保护作用(Zivcak et al. 2013)。干旱胁迫可增强PSII对热胁迫的抗性,表现为OJIP瞬态的K峰的消失(见图2c,Oukarroum et al. 2012)。ChlF方法可用于筛选耐旱性基因型(Guha et al. 2013)。OJIP荧光曲线上升的最初2~3ms阶段与初级光化学反应相关,Oukarroum et al. (2007)建议胁迫激发出现的L-band和K-band可作为评估应对和恢复干旱胁迫潜力的有效工具。L-band受PSII各组分间激发能传递的影响,通常表示为连通性(connectivity)或聚集性(grouping)(Strasser & Stirbet 1998)。不论是突变(Brestic et al. 2014)或环境条件(Zivcak et al. 2014a)而引发的PSII天线色素组分的改变,同样会使L-band受到影响。K-band的出现与放氧复合体OEC的解离有关(Guisse et al. 1995)。因此,O-L-K-J-I-P荧光瞬态的测量和利用JIP-test进行的分析可以作为干旱胁迫耐性和干旱胁迫可见症状出现前生理紊乱的有效指示工具。性能指数(PI)是应用最广泛的ChlF OJIP曲线参数,为我们提供了关于植物状态和活力的定量信息。PI由三个独立特性的参数乘积组成:每个叶绿素分反应中心浓度、初级光化学反应相关参数和电子传递相关参数(Strasser et al. 2004)。因此PI对天线特性、捕获效率或除QA外的电子传递的变化都很敏感。例如,冬小麦在花后长期干旱胁迫下,PI值降低。此外,根据干旱胁迫下的PI值估算的小麦基因型的耐旱性与以产量为指标的抗旱性也有很好的相关性(Zivcaket al. 2008)。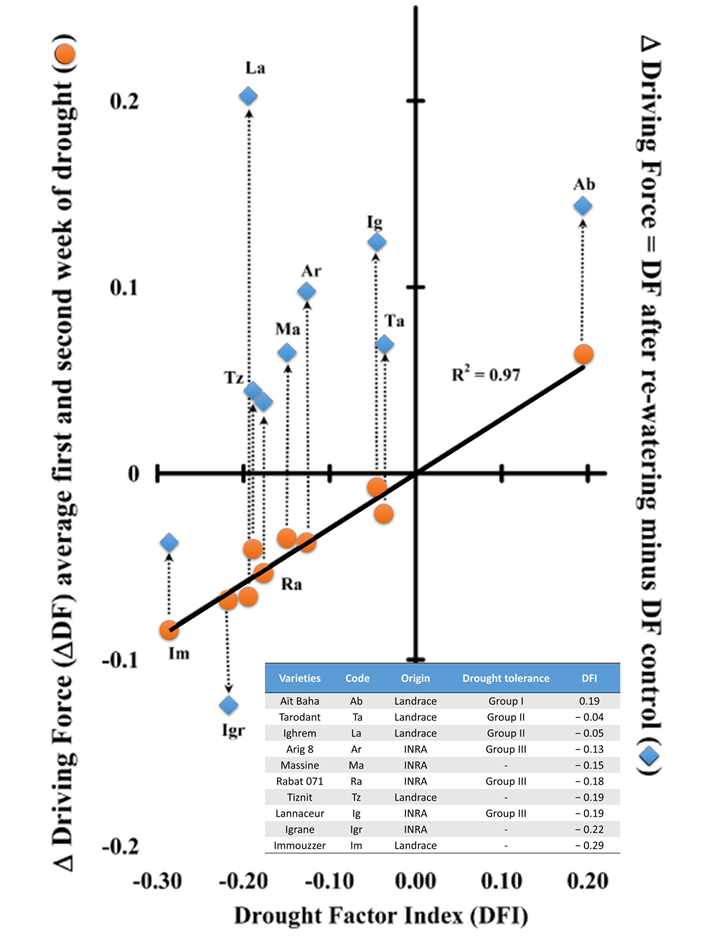
PI与干旱因子指数(DFI)密切相关,DFI表示在一定的干旱胁迫时间内,PI的相对减少量。Strauss等人使用了DFI方法(Strauss et al. 2006)评估不同大豆基因型的耐暗冷性。DFI还被用于对10个大麦品种(Oukarroum et al. 2007)和21种芝麻突变体种质资源(Boureima et al. 2012)的耐旱性进行排序。利用PI参数和ChlF快速诱导曲线成功筛选了来自埃及的**耐性和最敏感的大麦和高粱品种(Jedmowskiet al. 2013)。这些研究表明,在PSII水平上可以区分耐旱性和敏感性品种。在干旱胁迫下同样观察到ABS/RC的增大(Van Heerden et al. 2007; Gomeset al. 2012),这可能是由于部分PSIIRCs失活或天线尺寸增大而导致的。干旱胁迫也会影响OJIP曲线I~P相的相对振幅。I~P相是荧光曲线上升的最慢阶段(约30~200ms),与质体篮素PC和PSI中P700+的再还原有关(Schreiber et al. 1989; Schansker et al. 2003)。I~P相似乎与PSI反应中心的含量(Ceppiet al. 2012)或由820nm透射测量得到的线性电子传递活性(Zivcak et al. 2014a)有关。例如,不同大麦品种的I~P损失程度取决于它们的耐旱性(Oukarroum et al. 2009; Ceppi et al. 2012)。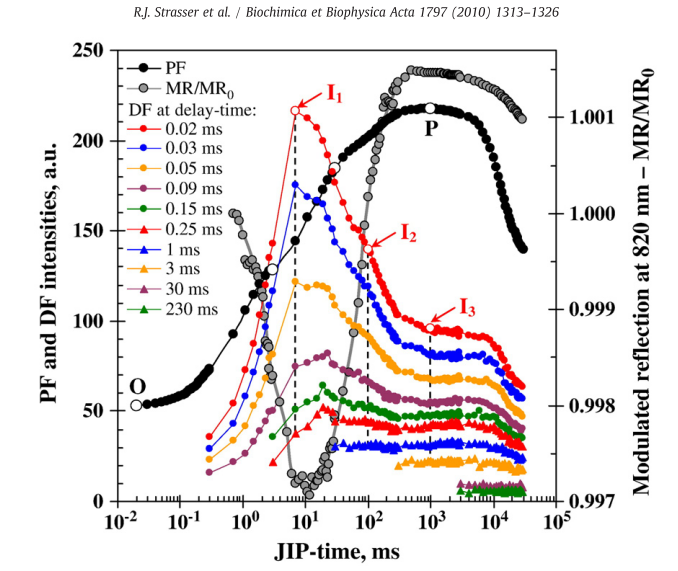
ChlF是在光合样品在暗到光转换之后发射的,而延迟荧光(DF)的发射发生在光到暗的转换过程中(Goltsev et al. 2009; Strasser et al. 2010; Kalaji et al. 2012)。DF被认为反映了还原的初级电子受体QA-和光诱导电荷分离形成的PSII氧化供体P680+的在黑暗状态下的再复合。DF诱导曲线的形状取决于样品材料类型和其生理状态。使用Hansatech公司M-PEA多功能植物效率分析仪同时测量ChlF 瞬时荧光OJIP曲线和DF曲线,目前被用于获取不同光合反映的速率常数(Strasser et al. 2010)。使用此技术Goltsev et al. (2012)观察到在干旱胁迫下QA-的再氧化受到抑制,PSII反应中心光诱导电子传递量子产率被抑制,调制反射信号(820nm)的光诱导动力学快速相降低。
植物对盐胁迫的反应是由多个方面决定的,如特定基因的表达、植物的发育阶段、甜菜碱的积累,这些甜菜碱通过稳定PSII复合体的外部蛋白质来保护光合机构(Murata et al. 1992)。盐分胁迫干扰了从RCs至质体醌库的电子传递(Strasser et al. 2000; 图2d)。Schreiber et al. (1994)鉴定出OEC是光合电子传递链中最敏感的组分之一。OEC性能的下降通常是由于电子传递紊乱引起的。盐胁迫下同样可观察到ChlF参数和PSII功能性的改变。在高盐胁迫下,由于LHC II和PSII解离导致了PSII反映中心捕获电子效率的下降(Havaux 1993)。在许多物种中,如大麦(Kalaji & Rutkowska 2004)、烟草Nicotiana tobacum L. (Yang et al. 2008)、甚至某些盐生植物如草珊瑚Sarcocorniafruticosa L中,均观察到了PSII最大量子效率(Fv/Fm)的降低和非光化学淬灭(NPQ或qN)的增加。此外,番茄和黄瓜幼苗在盐分胁迫下,光下PSII光化学效率(ΦPSII)、电子传递效率(ETR)和光照下PSII开放反应中心的效率均受影响而降低(He et al. 2009; Zhang & Sharkey 2009)。盐胁迫对小麦的伤害主要表现在供体侧,而非受体侧,这种损伤在PSII受体侧是完全可逆的(约100%),而供体侧的恢复率小于85%(Mehta et al. 2010b)。盐胁迫的渗透和离子效应也通过ChlF测量得到了有效区分(Singh-Tomar et al. 2012)。特定营养元素(N、P、K、Ca、Mg、S或Fe)的缺乏会破坏光合器官的功能,降低PSII光化学效率并改变ChlF参数的值(Smethurst et al. 2005)。氮素(N)缺乏是限制植物生长的关键因素,是所有蛋白质、核酸和其他有机化合物的组成部分。氮素缺乏导致类囊体膜的改变并扰乱其功能(图2e),并进一步加速叶绿体衰老和质体小球的形成(Wu et al. 2006)。氮也是RuBisCO光合复合物、卡尔文循环酶、叶绿素和类胡萝卜素中的重要元素(Correia et al. 2005)。氮缺乏会导致蒸腾作用、气孔导度、叶绿素和类胡萝卜素含量以及可溶性糖浓度的降低(Huang et al. 2004)。氮摄取不足也会降低PSII中的电子受体库,降低RuBisCO和磷酸烯醇式丙酮酸羧化酶(PEPCase)的活性(Correia et al. 2005)。JIP-test分析已经在处理氮缺乏的研究中多次应用,并且已经很好地描述了氮供应不足对PSII的影响(Redillas et al. 2011, Li et al. 2012)。特别是,氮素缺乏导致的反应中心密度显著降低(Dudeja & Chaudhary 2005)。同时,高氮处理对大豆(Van Heerden et al. 2004)、玉米(Li et al. 2012)和小麦(Zivcak et Val. 2014b)中PI值的积极影响已经得到了证明。磷对植物的生长发育也是必不可少的。磷缺乏将主要导致籽粒和类囊体膜结构改变、捕光复合体吸收PAR的下降,从而导致PSII活性的降低(Foyer & Spencer 1986)。磷缺乏还对NADPH的再生过程产生不利影响,降低光合作用的量子产率、羧化效率和电子传递效率(Wu et al. 2006)。JIP-test已成功应用于磷缺乏胁迫下植物PSII活性或效率的评估(Kruger et al. 1997; Tsimilli-Michael & Strasser 2008)。此外各种研究证明,JIP-test参数和气体交换或植物生长参数具有高度相关性(Strasser et al. 2000)。钾(K)在细胞渗透调节中起着关键作用:钾离子是保持类囊体膜上的pH梯度所必需的(Rampino et al. 2006)。钾缺乏会导致气孔导度阻力增加,限制二氧化碳通过气孔的扩散。在光合作用中,钾在许多酶的激活和ATP合成中的重要作用可能比它在调控气孔功能中的作用重要的多。然而钾缺乏对光合组织效率和PSII功能的影响知之甚少。然而,在缺钾条件下,一些光合参数如电子传递效率(ETR)和最大量子产率(Fv/Fm)都会降低(Schweiger et al. 1996)。有许多研究使用快速ChlF参数来分析其他矿物质缺乏对光化学功能的影响,例如钙(Liu et al. 2009; Lauriano et al. 2006)、镁(Smethurst et al. 2005)和铁(Molassiotis et al. 2006)。由于许多营养物质对PSII光化学反应有特殊的影响,这里的问题是是否有可能利用叶绿素荧光动力学来识别营养缺乏。尽管这一问题仍然悬而未决,Kalaji et al. (2014a, b)能够利用JIP-test参数的主成分分析(PCA)来识别番茄和玉米的主要营养素的缺乏情况。
高浓度的重金属胁迫会破坏光合作用进程,但特定重金属离子的影响可能是物种特异性的(Antosiewicz 2005; Mishra & Dubey 2005)。PSI被认为比PSII更能耐受重金属的胁迫(Romanowska et al. 2006; Tuba et al. 2010)。镉(Cd)是毒性最大的重金属之一,可在生物体内富集。环境中镉的来源包括磷肥和工业废料(Romanowska et al. 2006; Kalaji & Łoboda 2007)。然而,镉似乎不会影响光合色素的含量。对油菜幼苗的研究表明,在Cd存在下生长2周后,叶绿素a、叶绿素b和类胡萝卜素的含量没有显著变化(Janeczko et al. 2005)。然而,Cd确实对光合过程的光化学效率有负面影响。PSII比PSI对镉的影响更敏感,表明Cd以更大的强度破坏PSII功能(Mallick & Mohn 2003)。Cd同时影响PSII的供体和受体侧。在供体侧,它抑制OEC,而在受体侧,由于LHCII复合体的解离导致电子传递紊乱,抑制了QA-和QB-之间的电子传递(Sigfridsson et al. 2004)。Cd胁迫同样会引发非光学淬灭或热耗散的升高(Janeczko et al. 2005)。对油菜JIP-test参数的分析表明,Cd引起了油菜叶片横截面比能流如RC/CS、ETo/CS和OEC活性的降低(Janeczko et al. 2005)。PSII最大量子效率Fv/Fm是Cd胁迫影响最不敏感的参数。植物对镉的抗性与“清除”活性氧的能力、激活抗氧化酶[特别是过氧化物酶(Ekmekci et al. 2008)]以及合成抗氧化化合物[如谷胱甘肽(Streb et al. 2008)]等保护机制的启动有关。铅对植物也有有害影响。土壤和植物中铅的主要源头来自于燃煤发电厂、汽车尾气和工业废弃物(Mishra & Dubey 2005)。铅会引起呼吸代谢的改变,导致线粒体产生高能化合物,使ATP含量和ATP/ADP比值升高(Romanowska et al. 2002)。铅胁迫下植物光合作用效率的降低是由于叶绿体超微结构和类囊体膜脂成分的破坏,以及叶绿素和类胡萝卜素合成的减少造成的(Sharma & Dubey 2005)。铅胁迫会阻断如镁和铁等营养元素的吸收,而镁和铁是光合作用所必需的。此外铅还会导致OEC复合体的解离,并将Ca,Cl,Mn化合物从OEC复合体中分离去除(Sharma & Dubey 2005; Romanowska et al. 2006)。与对照组相比,暴露于铅胁迫的植物的O–J–I–P诱导曲线中I和P阶的荧光强度降低(图2f),并出现K峰(Kalaji & Łoboda 2007)。ChlF诱导曲线上出现以上变化可能与OEC和PSII反应中心之间的电子传递抑制有关(Strasser et al. 2004; Wu et al. 2008)。铅胁迫模型表明,PSII内的能量吸收和耗散很高,而电子捕获和电子传递则大幅减少(Lazár & Jablonsky 2009)。用于快速ChlF动力学分析的数学模型,如JIP-test,是专门用来评估微秒级或毫秒级叶绿体氧化还原反应级联反应的生物物理工具。尽管如此,早期的研究即已经获取了很多关于叶片生理状态和荧光瞬态曲线形状之间关系的有趣的经验理论(Strasser et al. 2000)。随后又有许多文献报道了叶片生理状态与ChlF瞬变之间的直接关系。而常被忽略的一个事实是测量得到的信号是一个复合信号(见上文关于PSII异质性的讨论),而该信号是与样品测定时的生理状态和环境条件高度相关的。因此,需要我们多方考虑各种因素的综合作用,以避免错误地或过度简化的得出结论(Evans 2009)。快速叶绿素荧光技术操作简单、快速,但如对其基本理论原理了解不深,很容易造成对该技术的不当应用。综合参数的使用,如性能指数(PI)可能比复杂的特定生物物理参数更有用,后者需要对光化学过程有更深入的理解才能正确地解释数据。Stirbet & Govindjee (2011)深入探讨了JIP-test在分析OJIP曲线中的各方利弊。为了避免ChlF应用中的错误,强烈建议所有用户熟悉该技术的各种理论细节(见Kalaji et al. 2014a综述文章)。本文介绍了叶绿素荧光技术在植物科学、农业和生态研究中应用的**信息。叶绿素荧光的测量信号及其统计分析(如JIP-test)可用于预测、监测和识别植物的胁迫。因此,它可以作为一种生物指示剂应用于几乎所有的植物生态学研究中。ChlF测量的多功能性意味着它们可以在单一植物的水平上应用于草原、农田甚至海洋生态系统。然而,这种潜在的多功能性强调了需要进行更实际和概念性的研究,使科学家能够获得有关植物生长和健康的可靠信息。这样一种方法不仅将使我们对光合作用的生理基础的理解得到改善,而且还将有助于了解和补救气候变化对作物产量和粮食安全的影响。
- AntosiewiczDM (2005) Study of calcium-dependent lead-tolerance on plants differing intheir level of Ca-deficiency tolerance. Environ Pollut 134(1):23–34
- BeddingtonJ, Asaduzzaman M, Clark M, Bremauntz AF, Guillou M, Howlett D, Jahn M, Lin E,Mamo T, Negra C (2012) What next for agriculture after Durban? Science335:289–290
- BernátG, Schreiber U, Sendtko E, Stadnichuk IN, Rexroth S, Rögner M, Koenig F (2012)Unique properties vs. common themes: the atypical cyanobacterium Gloeobacterviolaceus PCC 7421 is capable of state transitions and blue-light-inducedfluorescence quenching. Plant Cell Physiol 53(3):528–542
- BoureimaS, Oukarroum A, Diouf M, Cisse N, Van Damme P (2012) Screening for droughttolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophylla fluorescence. Environ Exp Bot 81:37–43
- BresticM, Zivcak M (2013) PSII Fluorescence techniques for measurement of drought andhigh temperature stress signal in crop plants: protocols and applications.Molecular stress physiology of plants. Springer, Berlin, pp 87–131
- BresticM, Zivcak M, Kalaji MH, Carpentier R, Allakhverdiev SI(2012) Photosystem IIthermostability in situ: environmentally induced acclimation andgenotype-specific reactions in Triticum aestivum L. Plant PhysiolBiochem 57:93–105
- BresticM, Zivcak M, Olsovska K, Repkova J (2013) Involvement of chlorophyll afluorescence analyses for identification of sensitiveness of the photosyntheticapparatus to high temperature in selected wheat genotypes. Photosynthesisresearch for food, fuel and the future. Springer, Berlin, pp 510–513
- BresticM, Zivcak M, Olsovska K, Shao HB, Kalaji MH, Allakhverdiev SI (2014) Reducedglutamine synthetase activity plays a role in control of photosyntheticresponses to high light in barley leaves. Plant Physiol Biochem 81:74–83.doi:10.1016/j.plaphy.2014.01.002
- CeppiMG, Oukarroum A, C ¸i c ¸ek N, Strasser RJ, Schansker G (2012) The IP amplitudeof the fluorescence rise OJIP is sensitive to changes in the photosystem Icontent of leaves: a study on plants exposed to magnesium and sulfatedeficiencies, drought stress and salt stress. Physiol Plant 144(3):277–288
- ChavesMM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress:regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560
- ChenLS, Li P, Cheng L (2009) Comparison of thermotolerance of sun-exposed peel andshaded peel of ‘Fuji’apple. Environ Exp Bot 66(1):110–116
- CorreiaCM, Pereira JMM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-Bradiation and nitrogen affect the photosynthesis of maize: a Mediterraneanfield study. Eur J Agron 22(3):337–347
- DudejaSS, Chaudhary P (2005) Fast chlorophyll fluorescence transient and nitrogenfixing ability of chickpea nodulation variants. Photosynthetica 43:253–259
- Ekmekc¸i Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme andphotosynthetic activities in leaves of two maize cultivars. J Plant Physiol165(6):600–611
- EvansJR (2009) Potential errors in electron transport rates calculated fromchlorophyll fluorescence as revealed by a multilayer leaf model. Plant CellPhysiology 50(4):698–706
- FoyerC, Spencer C (1986) The relationship between phosphate status andphotosynthesis in leaves. Planta 167(3):369–375
- GautumA, Agrawal D, SaiPrasad SV, Jajoo A (2014) A quick method to screen high andlow yielding wheat cultivars exposed to high temperature. Physiol Mol BiolPlants. doi:10.1007/s12298-014-0252-4
- GoltsevV, Zaharieva I, Chernev P, Kouzmanova M, Kalaji MH, Yordanov I, Krasteva V,Alexandrov V, Stefanov D, Allakh-verdiev SI, Strasser RJ (2012) Drought-inducedmodifications of photosynthetic electron transport in intact leaves: analysisand use of neural networks as a tool for a rapid non-invasive estimation.Biochim Biophys Acta 1817:1490–1498
- GoltsevV, Zaharieva I, Chernev P, Strasser RJ (2009) Delayed chlorophyll fluorescenceas a monitor for physiological state of photosynthetic apparatus. BiotechnolBiotechnol Equip 23(Special Edition):452–457
- GomesMTG, da Luz AC, dos Santos MR, Batitucci MDCP, Silva DM, Falqueto AR (2012)Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll afluorescence transient. Sci Hortic 142:49–56
- GottardiniE, Cristofori A, Cristofolini F, Nali C, Pellegrini E, Bussotti F, Ferretti M(2014) Chlorophyll-related indicators are linked to visible ozone symptoms:evidence from a field study on native Viburnum lantana L. plants innorthern Italy. Ecol Ind 39:65–74
- Govindjee(1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. AusJ Plant Physiol 22:131–160
- GuanterL, Zhang Y, Jung M, Joiner J, Voigt M, Berry JA, Frankenberg C, Huete AR,Zarco-Tejada P, Lee JE (2014) Global and time-resolved monitoring of cropphotosynthesis with chlorophyll fluorescence. Proc Natl Acad Sci111(14):E1327–E1333
- GuhaA, Sengupta D, Reddy AR (2013) Polyphasic chlorophyll a fluorescencekinetics and leaf protein analyses to track dynamics of photosyntheticperformance in mulberry during progressive drought. J Photochem Photobiol B119:71–83
- GuisséB, Srivastava A, Strasser RJ (1995) Effects of high temperature and waterstress on the polyphasic chlorophyll a fluorescence transient of potatoleaves. In: Mathis P (ed) Photosynthesis: from light to biosphere. KluwerAcademic Publishers, Dordrecht, pp 913–916
- HavauxM (1993) Rapid photosynthetic adaptation to heat stress triggered in potatoleaves by moderately elevated temperatures. Plant Cell Environ 16(4):461–467
- HeY, Zhu Z, Yang J, Ni X, Zhu B (2009) Grafting increases the salt tolerance oftomato by improvement of photosynthesis and enhancement of antioxidant enzymesactivity. Environ Exp Bot 66(2):270–278
- HuangZA, Jiang DA, Yang Y, Sun JW, Jin SH (2004) Effects of nitrogen deficiency ongas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves ofrice plants. Photosynthetica 42(3):357–364
- JajooA (2013) Changes in photosystem II in response to salt stress. Ecophysiologyand responses of plants under salt stress. Springer, Berlin, pp 149–168
- JaneczkoA, Koscielniak J, Pilipowicz M, Szarek-Lukaszewska G, Skoczowski A (2005)Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmiumstress. Photosynthetica 43(2):293–298
- JedmowskiC, Ashoub A, Bru ¨ggemann W (2013) Reactions of Egyptian landraces of Hordeumvulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fluorescencetransient analysis. Acta Physiologiae Plantarum 35(2):345–354
- KalajiMH, Rutkowska A (2004) Reactions of photosynthetic apparatus of maize seedlingsto salt stress. Zesz Probl Post NaukRol 496:545–558
- KalajiMH, Carpentier R, Allakhverdiev SI, Bosa K (2012) Fluorescence parameters asearly indicators of light stress in barley. J Photochem Photobiol B 112:1–6
- KalajiMH, Schansker G, Ladle RJ, et al (2014a) Frequently asked questions about invivo chlorophyll fluorescence: practical issues. Photosynth Res. doi:10.1007/s11120-014-0024-6
- KalajiMH, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Goltsev V(2014b) Identification of nutrient deficiency in maize and tomato plants by invivo chlorophyll a fluorescence measurements. Plant Physiol Biochem.doi:10.1016/j.plaphy.2014.03.029
- KautskyH, Hirsch A (1931) NeueVersuchezur Kohlensaureassimilation. Naturwissenschaften19:964
- KrugerGHJ, Tsimilli-Michael M, Strasser RJ (1997) Light stress provokes plastic andelastic modifications in structure and function of photosystem II in camellialeaves. Physiol Plant 101:265–277
- LaurianoJA, Ramalho JC, Lidon FC, Ce úmatos M (2006) Mechanisms of energy dissipationin peanut under water stress. Photosynthetica 44(3):404–410
- LazárD (2006) The polyphasic chlorophyll a fluorescence rise measured under highintensity of exciting light. Funct Plant Biol 33(1):9–30
- LazárD, Jablonsky J (2009) On the approaches applied in formulation of a kineticmodel of photosystem II: different approaches lead to different simulations ofthe chlorophyll a fluorescence transients. J Theor Biol 257(2):260–269
- LiG, Zhang ZS, Gao HY, Liu P, Dong ST, Zhang JW, Zhao B (2012) Effects ofnitrogen on photosynthetic characteristics of leaves from two differentstay-green corn (Zea mays L.) varieties at the grain-filling stage. CanJ Plant Sci 92:671–680
- LiuWJ, Chen YE, Tian WJ, Du JB, Zhang ZW, Xu F, Zhang F, Yuan S, Lin HH (2009)Dephosphorylation of photosystem II proteins and phosphorylation of CP29 inbarley photosynthetic membranes as a response to water stress. Biochim BiophysActa 1787(10):1238–1245
- LobellDB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL (2008)Prioritizing climate change adaptation needs for food security in 2030. Science319:607–610
- ŁobodaT, Kalaji MH (2007) Photosystem II of barley seedlings under cadmium and leadstress. Plant Soil Environ 53(12):511
- MalaspinaP, Giordani P, Faimali M, Garaventa F, Modenesi P (2014) Assessingphotosynthetic biomarkers in lichen transplants exposed under different lightregimes. Ecol Ind 43:126–131
- MallickN, Mohn F (2003) Use of chlorophyll fluorescence in metalstress research: acase study with the green microalga Scenedesmus. Ecotoxicol Environ Saf55(1):64–69
- MathurS, Mehta P, Jajoo A, Bharti S (2011a) Analysis of elevated temperature inducedinhibition of Photosystem II using Chl a fluorescence inductionkinetics. Plant Biology 13:1–6
- MathurS, Allakhverdiev SI, Jajoo A (2011b) Analysis of high temperature stress on thedynamics of antenna size and reducing side heterogeneity of Photosystem II inwheat leaves (Triticum aestivum). Biochim Biophys Acta 1807(1):22–29
- MathurS, Agrawal D, Jajoo A (2014) Photosynthesis: limitations in response to hightemperature stress. J Photochem Photobiol B Biol. doi: 10.1016/j.jphotobiol.2014.01.010
- MehtaP, Allakhverdiev SI, Jajoo A (2010a) Characterization of photosystem IIheterogeneity in response to high salt stress in wheat leaves (Triticumaestivum). Photosynth Res 105(3):249–255
- MehtaP, Jajoo A, Mathur S, Bharti S (2010b) Chlorophyll a fluorescence studyrevealing effects of high salt stress on Photosystem II in wheat leaves. PlantPhysiol Biochem 48(1):16–20
- MelisA, Homann PH (1976) Heterogeneity of the photochemical centers in system II ofchloroplasts. Photochem Photobiol 23:343–350
- MishraS, Dubey R (2005) Heavy metal toxicity induced alterations in photosyntheticmetabolism in plants. In: Pessarakli M (ed) Handbook of photosynthesis, vol 28.CRC Press, Boca Raton, pp 827–844
- MolassiotisA, Tanou G, Diamantidis G, Patakas A, Therios I (2006) Effects of 4-month Fedeficiency exposure on Fe reduction mechanism, photosynthetic gas exchange,chlorophyll fluorescence and antioxidant defense in two peach rootstocksdiffering in Fe deficiency tolerance. J Plant Physiol 163(2):176–185
- MurataN, Mohanty P, Hayashi H, Papageorgiou G (1992) Glycinebetaine stabilizes theassociation of extrinsic proteins with the photosynthetic oxygen-evolvingcomplex. FEBS Lett 296(2):187–189
- OukarroumA, Madidi SE, Schansker G, Strasser RJ (2007) Probing the responses of barleycultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIPunder drought stress and rewatering. Environ Exp Bot 60(3):438–446
- OukarroumA, Schansker G, Strasser RJ (2009) Drought stress effects on photosystem Icontent and photosystem II thermotolerance analyzed using Chl afluorescence kinetics in barley varieties differing in their drought tolerance.Physiol Plant 137(2):188–199
- OukarroumA, El Madidi S, Strasser RJ (2012) Exogenous glycine betaine and proline play aprotective role in heat-stressed barley leaves (Hordeum vulgare L.): achlorophyll a fluorescence study. Plant Biosyst 146(4):1037–1043
- PapageorgiouGC, Govindjee (2011) Photosystem II fluorescence: slow changes—scaling from thepast. J Photochem Photobiol B 104(1–2):258–270
- RampinoP, Pataleo S, Gerardi C, Mita G, Perrotta C (2006) Drought stress response inwheat: physiological and molecular analysis of resistant and sensitivegenotypes. Plant Cell Environ 29(12):2143–2152
- RedillasMCFR, Jeong JS, Strasser RJ, Kim YS, Kim JK (2011) JIP analysis on rice (Oryzasativa cv Nipponbare) grown under limited nitrogen conditions. J Korean SocAppl Biol Chem 54:827–832
- RomanowskaE, Igamberdiev AU, Parys E, Gardeström P (2002) Stimulation of respiration byPb2+ in detached leaves and mitochondria of C3 and C4 plants.Physiol Plant 116(2):148–154
- RomanowskaE, Wróblewska B, Dro_ zak A, Siedlecka M (2006) High light intensity protects photosyntheticapparatus of pea plants against exposure to lead. Plant Physiol Biochem44(5):387–394
- SchanskerG, Srivastava A, Govindjee, Strasse RJ (2003) Characterization of the 820-nmtransmission signal paralleling the chlorophyll a fluorescence rise (OJIP)in pea leaves. Funct Plant Biol 30(7):785–796
- SchreiberU, Neubauer C, Klughammer C (1989) Devices and methods for room-temperaturefluorescence analysis. Philos Trans Royal Soc London B Biol Sci323(1216):241–251
- SchreiberU, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusiveindicator for rapid assessment of in vivo photosynthesis. Ecophysiology ofphotosynthesis. Springer, Berlin, pp 49–70
- SchreiberU, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parametersof photosynthetic electron transport with a new type of multi-color PAMchlorophyll fluorometer. Photosynth Res 113(1–3):127–144
- SchweigerJ, Lang M, Lichtenthaler HK (1996) Differences in fluorescence excitationspectra of leaves between stressed and non-stressed plants. J Plant Physiol148(5):536–547
- SharkeyTD, Schrader SM (2006) High temperature stress. Physiology and molecularbiology of stress tolerance in plants. Springer, Berlin, pp 101–129
- SharmaP, Dubey RS (2005) Lead toxicity in plants. Brazilian Journal of PlantPhysiology 17:35–52
- SigfridssonKG, Berna ′t G, Mamedov F, Styring S (2004) Molecular interference of Cd2+with Photosystem II. Biochim Biophys Acta 1659(1):19–31
- Singh-TomarR, Mathur S, Allakhverdiev SI, Jajoo A (2012) Changes in PSII heterogeneity inresponse to osmotic and ionic stress in wheat leaves (Triticum aestivum). JBioenerg Biomembr 44:411–419
- SmethurstCF, Garnett T, Shabala S (2005) Nutritional and chlorophyll fluorescenceresponses of lucerne (Medicago sativa) to waterlogging and subsequent recovery.Plant Soil 270(1):31–45
- SrivastavaA, Strasser RJ (1995) How do land plants respond to stress temperature andstress light? Arch Sci Geneve 48:135–146
- SrivastavaA, Strasser RJ, Govindjee (1999) Greening of peas: parallel measurements of 77K emission spectra, OJIP chlorophyll a fluorescence transient, periodfour oscillation of the initial fluorescence level, delayed light emission, andP700. Photosynthetica 37:365–392
- StefanovD, Petkova V, Denev ID (2011) Screening for heat tolerance in common bean (Phaseolusvulgaris L.) lines and cultivars using JIP-test. Sci Hortic 128(1):1–6
- StirbetA (2013) Excitonic connectivity between photosystem II units: what is it, andhow to measure it? Photosynth Res 116:189–214
- StirbetA, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll afluorescence induction) and Photosystem II: basics and applications of the OJIPfluorescence transient. J Photochem Photobiol B 104(1–2):236–257
- StirbetA, Govindjee (2012) Chlorophyll a fluorescence induction: a personalperspective of the thermal phase, the J–I–P rise. Photosynth Res 113:15–61
- StrasserRJ, Stirbet AD (1998) Heterogeneity of photosystem II probed by the numericallysimulated chlorophyll a fluorescence rise (O–J–I–P). Math Comput Simul48(1):3–9
- StrasserRJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as atool to characterize and screen photosynthetic samples. In: Mohanty P, Yunus,Pathre (eds) Probing photosynthesis: mechanism, regulation and adaptation.Taylor and Francis, London, pp 443–480
- StrasserRJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll afluorescence transient. In: Papageorgiou G, Govindjee (eds) Advances inphotosynthesis and respiration. chlorophyll a fluorescence: a signatureof photosynthesis. Springer, Dordrecht, pp 321–362
- StrasserRJ, Tsimilli-Michael M, Srivastava A, Srivastava A (2005) Analysis of thechlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee(eds) Advances in photosynthesis and respiration. Chlorophyll afluorescence: a signature of photosynthesis. Kluwer Acad. Publ, Dordrecht, pp321–362
- StrasserRJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivorecording of prompt and delayed fluorescence and 820-nm reflection changesduring drying and after rehydration of the resurrection plant Haberlearhodopensis. Biochimical BiophysicalActa 1797:1313–1326
- StraussA, Kru ¨ger G, Strasser RJ, Heerden PV (2006) Ranking of dark chillingtolerance in soybean genotypes probed by the chlorophyll a fluorescencetransient OJIP. Environ Exp Bot 56(2):147–157
- StrebP, Aubert S, Gout E, Feierabend J, Bligny R (2008) Cross tolerance toheavy-metal and cold-induced photoinhibiton in leaves of Pisum sativumacclimated to low temperature. Physiol Mole Biol Plants 14(3):185–193
- SuzukiK, Ohmori Y, Ratel E (2011) High root temperature blocks both linear and cyclicelectron transport in the dark during chilling of the leaves of rice seedlings.Plant Cell Physiology 52(9):1697–1707
- TomarRS, Jajoo A (2013) A quick investigation of the detrimental effects ofenvironmental pollutant polycyclic aromatic hydrocarbon fluoranthene on thephotosynthetic efficiency of wheat (Triticum aestivum). Ecotoxicology22(8):1313–1318
- TomarRS, Jajoo A (2014) Fluoranthene, a polycyclic aromatic hydrocarbon, inhibitslight as well as dark reactions of photosynthesis in wheat (Triticum aestivum).Ecotoxicol Environ Saf 109:110–115
- Tsimilli-MichaelM, Strasser RJ (2008) In vivo assessment of stress impact on plants’ vitality:applications in detecting and evaluating the beneficial role of Mycorrhizationon host plants. In: Varma A (ed) Mycorrhiza: state of the art, genetics and molecularbiology, eco-function, biotechnology, eco-physiology, structure andsystematics, vol 3rd. Springer, Berlin, pp 679–703
- Tsimilli-MichaelM, Strasser RJ (2013) The energy flux theory 35 years later: formulations andapplications. Photosynth Res 117(1–3):289–320. doi:10.1007/s11120-013-9895-1
- TubaZ, Saxena DK, Srivastava K, Singh S, Czebol S, Kalaji MH (2010) Chlorophyll afluorescence measurements for validating the tolerant bryophytes for heavymetal (Pb) biomapping. Curr Sci 98(11):1505–1508
- VanHeerden PD, Strasser RJ, Kru ¨ger GH (2004) Reduction of dark chilling stressin N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescencekinetics. Physiol Plant 121:239–249
- VanHeerden PDR, Swanepoel JW, Kru ¨ger GHJ (2007) Modulation of photosynthesis bydrought in two desert scrub species exhibiting C3-mode CO2 assimilation.Environ Exp Bot 61(2):124–136
- WuC, Wang Z, Sun H, Guo S (2006) Effects of different concentrations of nitrogenand phosphorus on chlorophyll biosynthesis, chlorophyll a fluorescence,and photosynthesis in Larix olgensis seedlings. Front Forest China1(2):170–175
- WuX, Hong F, Liu C, Su M, Zheng L, Gao F, Yang F (2008) Effects of Pb2+on energy distribution and photochemical activity of spinach chloroplast.Spectrochimica Acta Part A Mol Biomol Spectrosc 69(3):738–742
- YamaneY, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo leveland reversible inhibition of Photosystem II reaction center by high-temperaturetreatments in higher plants. Photosynth Res 52:57–64
- YangX, Liang Z, Wen X, Lu C (2008) Genetic engineering of the biosynthesis ofglycinebetaine leads to increased tolerance of photosynthesis to salt stress intransgenic tobacco plants. Plant Mol Biol 66(1–2):73–86
- YangJ, Kong Q, Xiang C (2009) Effects of low night temperature on pigments, chl afluorescence and energy allocation in two bitter gourd (Momordica charantiaL.) genotypes. Acta Physiologiae Plantarum 31(2):285–293
- ZhangR, Sharkey TD (2009) Photosynthetic electron transport and proton flux undermoderate heat stress. Photosynth Res 100(1):29–43
- ZivcakM, Brestic M, Olsovska K, Slamka P (2008) Performance index as a sensitiveindicator of water stress in Triticum aestivum. Plant Soil Environ54:133–139
- ZivcakM, Brestic M, Balatova Z, Drevenakova P, Olsovska K, Kalaji MH, AllakhverdievSI (2013) Photosynthetic electron transport and specific photoprotectiveresponses in wheat leaves under drought stress. Photosynth Res 117:529–546
- ZivcakM, Kalaji MH, Shao HB, Olsovska K, Brestic M (2014). Photosynthetic proton andelectron transport in wheat leaves under prolonged moderate drought stress. JPhotochem Photobiol B Biol. doi: 10.1016/j.jphotobiol.2014.01.007
- ZivcakM, Olsovska K, Slamka P, Galambosova J, Rataj V, Shao HB, Brestic M (2014b)Application of chlorophyll fluorescence performance indices to assess the wheatphotosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ60:210–215
- ZushiK, Kajiwara S, Matsuzoe N (2012) Chlorophyll a fluorescence OJIP transient as atool to characterize and evaluate response to heat and chilling stress intomato leaf and fruit. Sci Hortic 148:39–46
|
|

 CIRAS4第四代便携式光合作用测定系统
CIRAS4第四代便携式光合作用测定系统
 FMS-300脉冲调制式荧光仪
FMS-300脉冲调制式荧光仪
 液相氧电极
液相氧电极
 CFLUX-1全自动土壤呼吸测定仪
CFLUX-1全自动土壤呼吸测定仪
 ——EGM-5 土壤碳通量快速测定
——EGM-5 土壤碳通量快速测定
 M-PEA 多功能植物效率分析仪
M-PEA 多功能植物效率分析仪
 TARGAS-1 便携式光合仪
TARGAS-1 便携式光合仪
 ——CFLUX-1全自动土壤呼吸测定仪
——CFLUX-1全自动土壤呼吸测定仪
 ——LSI-LASTEM气象监测系统
——LSI-LASTEM气象监测系统
 ——科研前线
——科研前线




